

Our COA: Includes three layers of analysis:
Chemical analysis (identity, purity, quantity, pH)
Heavy metals screening (arsenic, cadmium, lead, antimony – all non-detect)
Microbial testing – not just E. coli and Salmonella but a full microbial panel of >30 organisms, including fungi like Aspergillus, Candida, and pathogens like Listeria, Pseudomonas, and Clostridium.
Typical competitors: Often limit testing to purity (HPLC) and maybe heavy metals. Microbial testing is frequently skipped or limited to USP-required basics.
Why it matters: This COA provides pharmaceutical-grade confidence, while most others only prove purity.


This COA: Signed off by an Analytical Chemist, plus lists an MD Laboratory Director, Clinical Director, and PhD Operations Director.
Typical competitors: Usually just “Lab Manager” or no names at all.
Why it matters: Adds credibility and accountability, making it closer to clinical testing standards.


Our COA: Provides explicit disclaimers:
Not FDA-approved.
For research use only, not human/veterinary.
Clear explanation of limitations of microbial detection
Typical competitors: Many omit disclaimers entirely or bury them in fine print.
Why it matters: Shows compliance, honesty, and a higher trust factor.
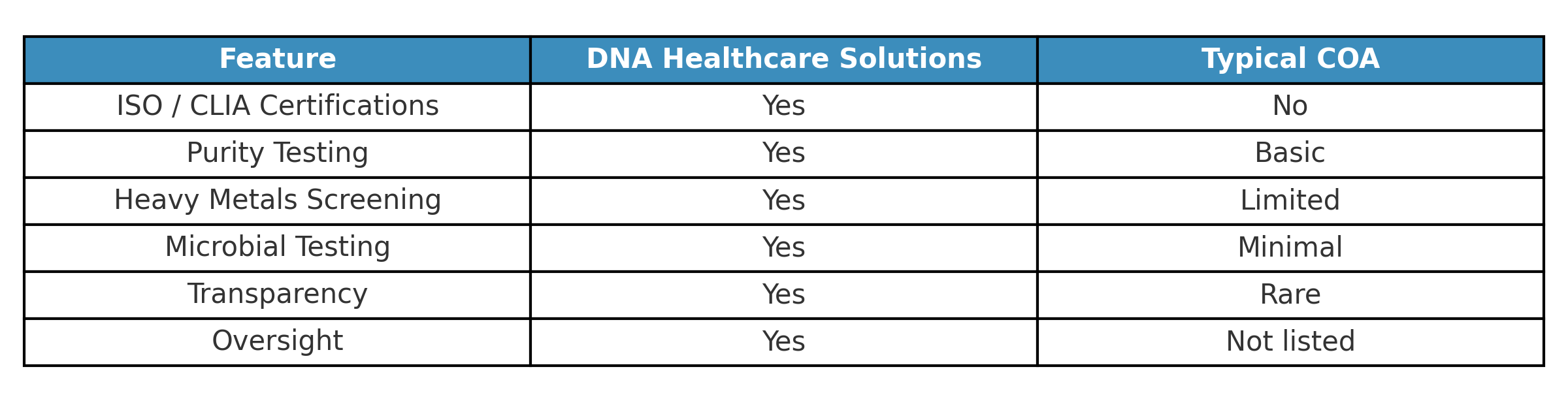
ISO + CLIA certified lab (rare in this industry).
Full microbial panel (far beyond standard COAs).
Heavy metals all non-detect.
Transparency of lab methodology.
Multiple MDs and PhDs overseeing results.
Clear legal compliance statements.
